

This property can be used to find the internuclear distance between two molecules forming a single covalent bond among themselves. Hence crystal radius of oxygen = 132 / 2 = 66 pm. the distance between two oxygen atoms in molecular oxygen is 132 pm. Hence crystal radius of sodium = 372 / 2 = 186 pm.Ĭovalent radius is defined as one half of the distance between the centres of the two similar nuclei of two similar atoms bonded together by a single covalent bond. For e.g. the distance between two sodium atoms in a sodium crystal is 372 pm. It is defined as one half of the distance between the centres of nuclei of two adjacent atoms in a metallic crystal. Other Terms Related to Atomic Radius: Crystal Radius: Hence the definition given in above point is arbitrary.
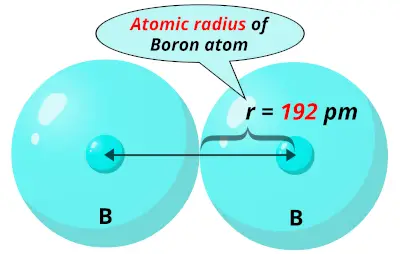
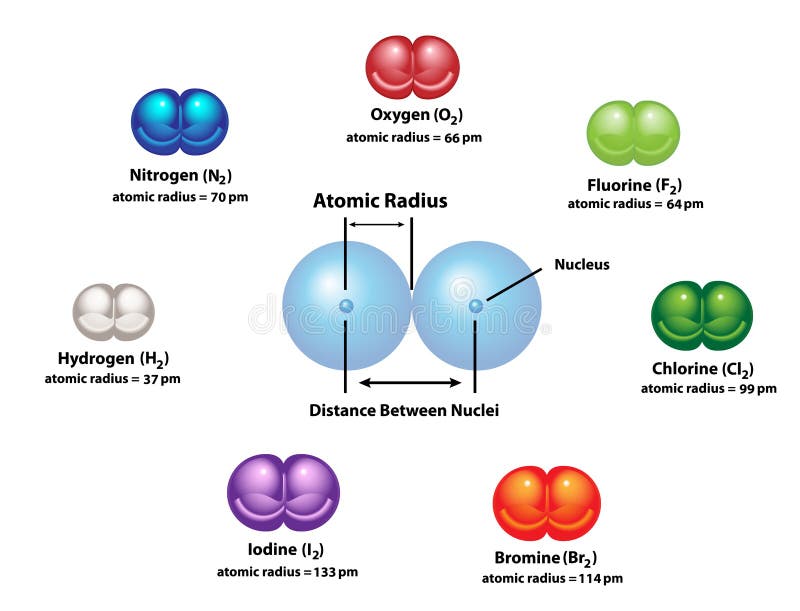
The electron density in an atom is greatly influenced by the presence of other atoms around the bonding atom and the nature (type) of bonding with neighbouring atoms, Depending upon this the terms like crystal radius, covalent radius, van der Walls’ radius, tetrahedral radius, etc. The atomic radius (atomic size) may be regarded as the distance from the centre of the atom to the outermost (valence) shell of electrons. One practical approach of finding the size of an atom of a non-metallic element is to measure the distance between two atoms when they are bound together by a single bond in a covalent molecule and from this value, the “Covalent Radius” of the element can be calculated. Secondly, since the electron cloud surrounding the atom does not have a sharp boundary, the determination of the atomic size cannot be precise. This means that #"Al"#'s atomic radius will be smaller than that of #"K"#, but bigger than that of #"C"# and #"O"#.The size of an atom is very small (120 pm). Another reason for the increase in atomic radius is the electron shielding effect, which states that the electrons in the higher energy levels are being shielded by those closer to the nucleus, further reducing the nucleus' pull on them. This happens because electrons are now being added to higher energy levels, further away from the nucleus, which weakens the nucleus' pull. As you move down a colomn (a group) of the periodic table, atomic radius increases. This is where the other periodic trends comes into play. Since #"C"# comes before #"O"# in the second period, #"O"# will have a smaller atomic radius than #"C"#.Īs mentioned before, #"Al"# and #"K"# don't share a period. We know that atomic radius decreases from left to right within a period due to the increase in effective nuclear charge.Īs you move to the right within a period, the number of protons elements have increases simultaneously with the number of electrons however, electrons are being added to the same energy level across a period, which means that the increasing number of protons allows the nucleus to exert more pull on these electrons, which in turn makes the atomic radius smaller. This aspect will allow you to easily rank them with respect to increasing atomic radius, since both of the periodic trends in atomic size are on display here. The correct order with respect to increasing atomic radius isĪn important thing to notice about these four elements is the fact that only two of them, #"C"# and #"O"#, are in the same row (the same period) of the periodic table the other two, #"Al"# and #"K"#, don't share a period neither with #"C"# and #O#, nor with each other, as #"Al"# is in the third period and #"K"# is in the fourth.


 0 kommentar(er)
0 kommentar(er)
